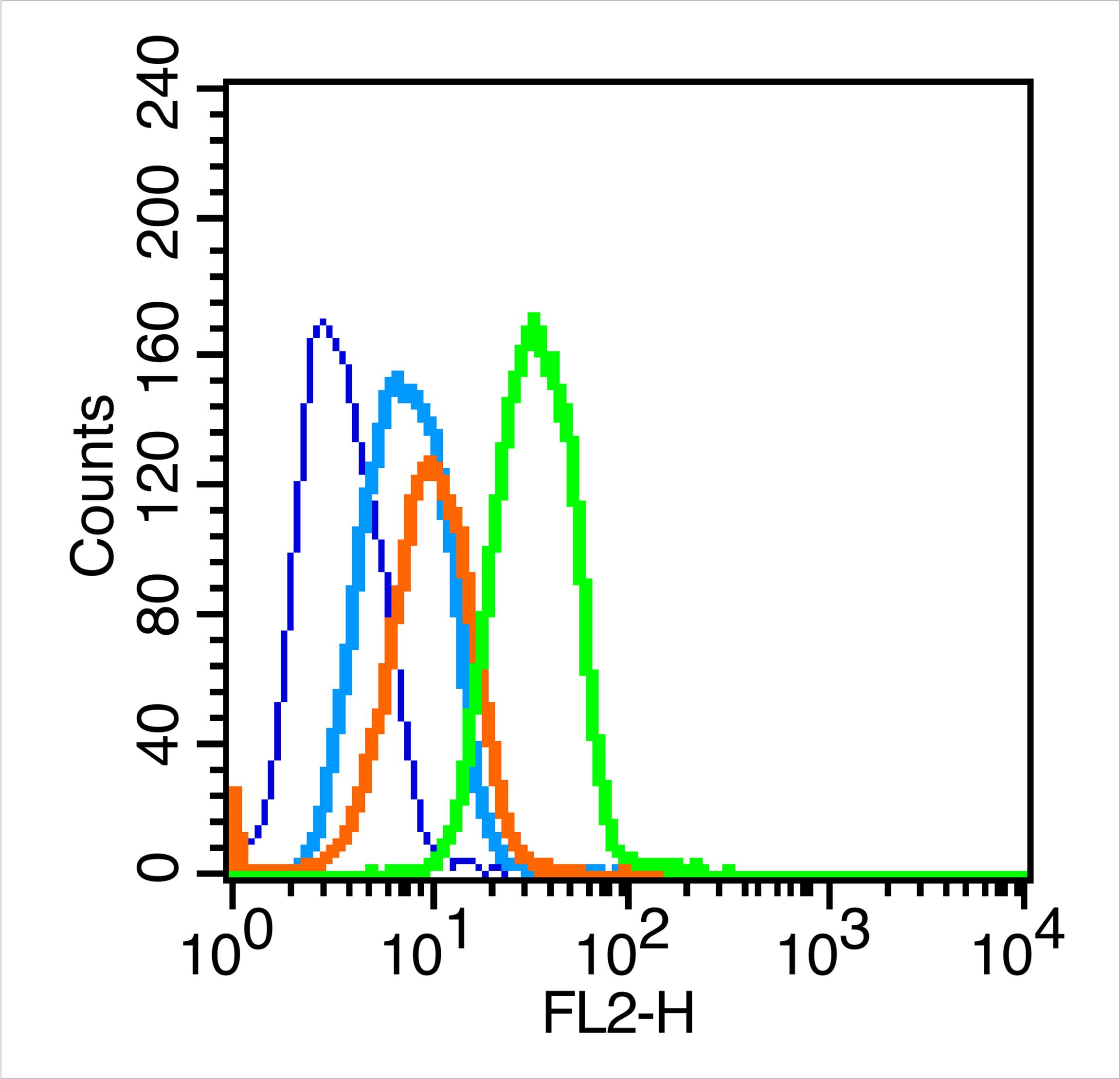
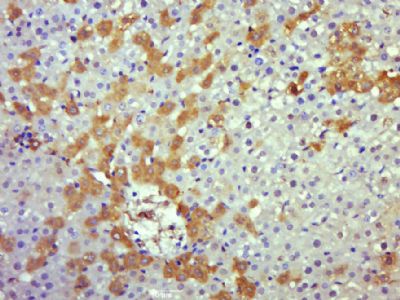
Caspase 8 Rabbit pAb
Caspase 8 Rabbit pAb
- 产品详情
- 实验流程
- 背景知识
Application
| WB, IHC-P, IHC-F, IF |
|---|---|
| Primary Accession | Q9JHX4 |
| Reactivity | Human, Rat |
| Predicted | Mouse, Dog, Pig, Horse |
| Host | Rabbit |
| Clonality | Polyclonal |
| Calculated MW | 55339 Da |
| Physical State | Liquid |
| Immunogen | KLH conjugated synthetic peptide derived from rat Caspase-8 subunit p10 |
| Epitope Specificity | 411-482/482 |
| Isotype | IgG |
| Purity | affinity purified by Protein A |
| Buffer | 0.01M TBS (pH7.4) with 1% BSA, 0.02% Proclin300 and 50% Glycerol. |
| SUBCELLULAR LOCATION | Cytoplasm. |
| SIMILARITY | Belongs to the peptidase C14A family.Contains 2 DED (death effector) domains. |
| SUBUNIT | Heterotetramer that consists of two anti-parallel arranged heterodimers, each one formed by a 18 kDa (p18) and a 10 kDa (p10) subunit. Interacts with FADD, CFLAR and PEA15. Isoform 9 interacts at the endoplasmic reticulum with a complex containing BCAP31, BAP29, BCL2 and/or BCL2L1. Interacts with TNFAIP8L2. |
| DISEASE | Defects in CASP8 are the cause of caspase-8 deficiency (CASP8D) [MIM:607271]. CASP8D is a disorder resembling autoimmune lymphoproliferative syndrome (ALPS). It is characterized by lymphadenopathy, splenomegaly, and defective CD95-induced apoptosis of peripheral blood lymphocytes (PBLs). It leads to defects in activation of T-lymphocytes, B-lymphocytes, and natural killer cells leading to immunodeficiency characterized by recurrent sinopulmonary and herpes simplex virus infections and poor responses to immunization. |
| Important Note | This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications. |
| Background Descriptions | Caspases are cysteine proteases, expressed as inactive precursors, that mediate apoptosis by proteolysis of specific substrates. Caspases have the ability to cleave after aspartic acid residues. There are two classes of caspases involved in apoptosis; initiators (activation by receptor cluster) and effectors (activation by mitochondrial permeability transition). Proapoptotic signals autocatalytically activate initiator caspases, such as Caspase 8 and Caspase 9. Activated initiator caspases then process effector caspases, such as Caspase 3 and Caspase 7, which in turn cause cell collapse. |
| Gene ID | 64044 |
|---|---|
| Other Names | Caspase-8 {ECO:0000303|PubMed:10197541, ECO:0000303|Ref.2}, CASP-8, 3.4.22.61, Casp8 {ECO:0000303|PubMed:10197541, ECO:0000312|RGD:620945} |
| Target/Specificity | Isoform 1, isoform 5 and isoform 7 are expressed in a wide variety of tissues. Highest expression in peripheral blood leukocytes, spleen, thymus and liver. Barely detectable in brain, testis and skeletal muscle. |
| Dilution | WB=1:500-2000,IHC-P=1:100-500,IHC-F=1:100-500,IF=1:100-500,Flow-Cyt=1 µg /Test |
| Storage | Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C. |
| Name | Casp8 {ECO:0000303|PubMed:10197541, ECO:0000312|RGD:620945} |
|---|---|
| Function | Thiol protease that plays a key role in programmed cell death by acting as a molecular switch for apoptosis, necroptosis and pyroptosis, and is required to prevent tissue damage during embryonic development and adulthood (By similarity). Initiator protease that induces extrinsic apoptosis by mediating cleavage and activation of effector caspases responsible for FAS/CD95-mediated and TNFRSF1A- induced cell death (PubMed:10197541). Cleaves and activates effector caspases CASP3, CASP4, CASP6, CASP7, CASP9 and CASP10 (By similarity). Binding to the adapter molecule FADD recruits it to either receptor FAS/CD95 or TNFRSF1A (PubMed:10197541). The resulting aggregate called the death-inducing signaling complex (DISC) performs CASP8 proteolytic activation (By similarity). The active dimeric enzyme is then liberated from the DISC and free to activate downstream apoptotic proteases (By similarity). Proteolytic fragments of the N-terminal propeptide (termed CAP3, CAP5 and CAP6) are likely retained in the DISC (By similarity). Also cleaves and activates BID, thereby promoting cytochrome C release from mitochrondria (By similarity). In addition to extrinsic apoptosis, also acts as a negative regulator of necroptosis: acts by cleaving RIPK1 at 'Asp-325', which is crucial to inhibit RIPK1 kinase activity, limiting TNF-induced apoptosis, necroptosis and inflammatory response (By similarity). Also able to initiate pyroptosis by mediating cleavage and activation of gasdermin-C and -D (GSDMC and GSDMD, respectively): gasdermin cleavage promotes release of the N-terminal moiety that binds to membranes and forms pores, triggering pyroptosis (By similarity). Initiates pyroptosis following inactivation of MAP3K7/TAK1 (By similarity). Also acts as a regulator of innate immunity by mediating cleavage and inactivation of N4BP1 downstream of TLR3 or TLR4, thereby promoting cytokine production (By similarity). May participate in the Granzyme B (GZMB) cell death pathways (By similarity). Cleaves PARP1 and PARP2 (By similarity). |
| Cellular Location | Cytoplasm. Nucleus |
For Research Use Only. Not For Use In Diagnostic Procedures.
Provided below are standard protocols that you may find useful for product applications.
BACKGROUND
Caspases are cysteine proteases, expressed as inactive precursors, that mediate apoptosis by proteolysis of specific substrates. Caspases have the ability to cleave after aspartic acid residues. There are two classes of caspases involved in apoptosis; initiators (activation by receptor cluster) and effectors (activation by mitochondrial permeability transition). Proapoptotic signals autocatalytically activate initiator caspases, such as Caspase 8 and Caspase 9. Activated initiator caspases then process effector caspases, such as Caspase 3 and Caspase 7, which in turn cause cell collapse.
REFERENCES
Sakamaki K.,et al.Eur. J. Biochem. 253:399-405(1998).
Van de Craen M.,et al.J. Mol. Biol. 284:1017-1026(1998).
Kioschis P.,et al.Submitted (JUL-1997) to the EMBL/GenBank/DDBJ databases.
Milovic-Holm K.,et al.EMBO J. 26:391-401(2007).
Villen J.,et al.Proc. Natl. Acad. Sci. U.S.A. 104:1488-1493(2007).
终于等到您。ABCEPTA(百远生物)抗体产品。
点击下方“我要评价 ”按钮提交您的反馈信息,您的反馈和评价是我们最宝贵的财富之一,
我们将在1-3个工作日内处理您的反馈信息。
如有疑问,联系:0512-88856768 tech-china@abcepta.com.























 癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。
癌症的基本特征包括细胞增殖、血管生成、迁移、凋亡逃避机制和细胞永生等。找到癌症发生过程中这些通路的关键标记物和对应的抗体用于检测至关重要。 为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。
为您推荐一个泛素化位点预测神器——泛素化分析工具,可以为您的蛋白的泛素化位点作出预测和评分。 细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。
细胞自噬受体图形绘图工具为你的蛋白的细胞受体结合位点作出预测和评分,识别结合到自噬通路中的蛋白是非常重要的,便于让我们理解自噬在正常生理、病理过程中的作用,如发育、细胞分化、神经退化性疾病、压力条件下、感染和癌症。